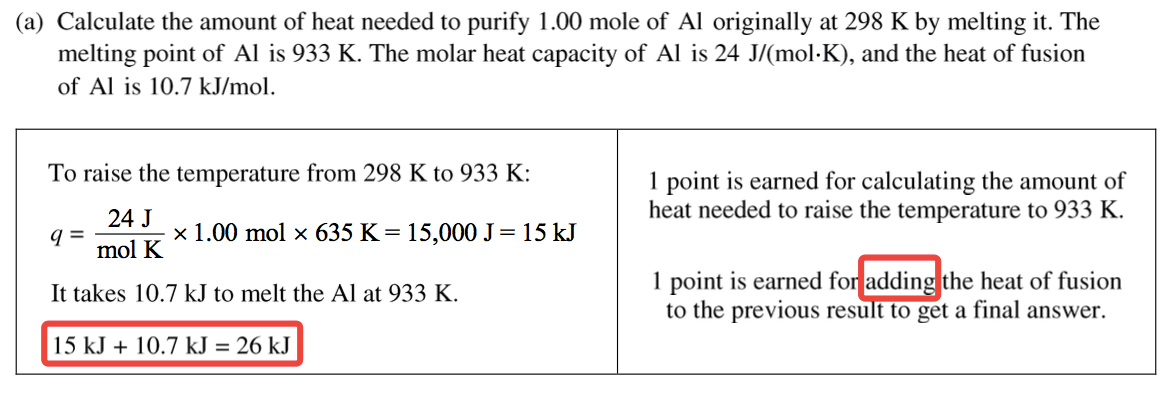
Question 1 (c)

![The Nernst Equation The effect of concentration on cell emf can be
obtained from the effect of concentra- tion on free-energy change. ax:
(Section 19.7) Recall that the free-energy change for any chemical
reaction, AG, is related to the standard free-energy change for the
reac- tion, AGO: AG = AGO + RT1nQ \[20.15\] The quantity Q is the
reaction quotient, which has the form of the equilibrium-constant
expression except that the concentrations are those that exist in the
reaction mixture at ex-x-a (Section 15.6) a given moment. Substituting
AG = —nFE (Equation 20.11) into Equation 20.15 gives nFE — -nFEO +
RT1nQ Solving this equation for E gives the Nernst equation: In Q This
equation is customarily expressed in terms of the base-IO logarithm:
2.303 RT log Q \[20.16\] \[20.17\] At T = 298 K, the quantity 2.303
RT/F equals 0.0592, with units of volts, and so the Nernst equation
simplifies to 0.0592 v logQ (T = 298 K) n \[20.18\]](media/image229.png)
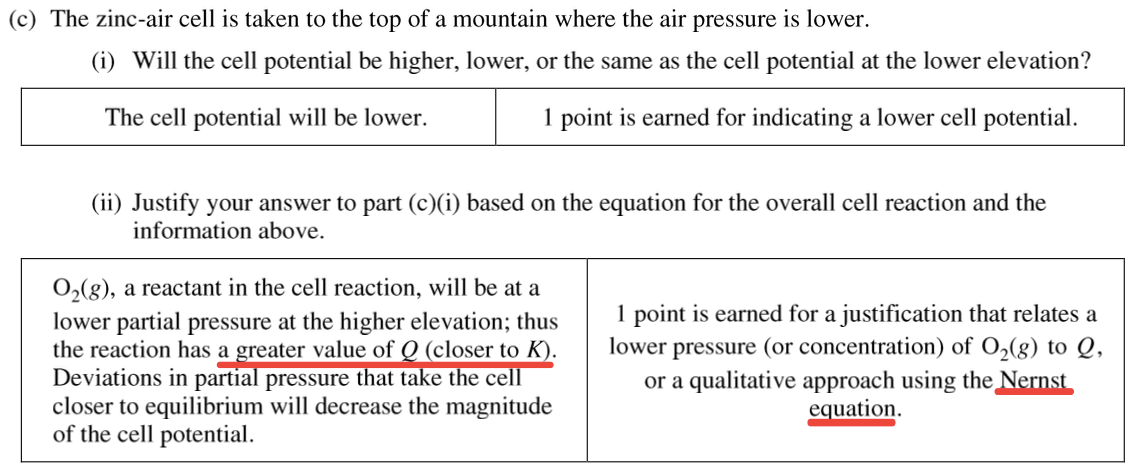
Question 2 (c)
![Relationship between AGO and K We can now use Equation 19.19 to
derive the relationship between AGO and the equi- librium constant, K.
At equilibrium, AG = 0 and Q = K. Thus, at equilibrium, Equa- tion
19.19 transforms as follows: AG = AGO + RT1nQ O = AGO + RT1nK AGO =
-RT1nK \[19.20\] Equation 19.20 is a very important one, with broad
significance in chemistry. By relating K to AGO, we can also relate K
to entropy and enthalpy changes for a reaction.](media/image230.png)
![We can also solve Equation 19.20 for K, to yield an expression that
allows us to calculate K if we know the value of AGO: AGO In K -RT
-AGO/RT \[19.21\] As usual, we must be careful in our choice of units.
In Equations 19.20 and 19.21 we again express AGO in kJ/mol. In the
equilibrium-constant expression, we use atmospheres for gas pressures,
molarities for solutions; and solids, liquids, and solvents do not
appear in the expression. (Section 15.4) Thus, the equi- librium
constant is KP for gas-phase reactions and Kc for reactions in
solution. Section 15.2 From Equation 19.20 we see that if AGO is
negative, In K must be positive, which eans K > 1. Therefore, the
more negative AGO is, the larger K is. Conversely, if AGO is ositive,
In K is negative, which means K < 1. Finally, if AGO is zero, K = 1.](media/image231.png)
Question 2 (e)

Question 2 (f)
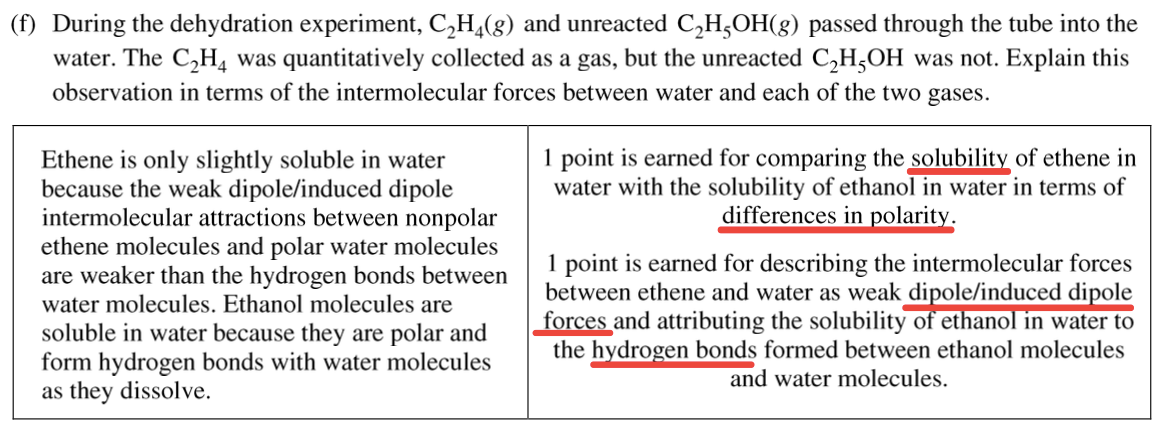
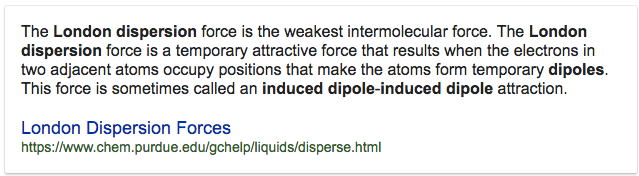
Question 4 (b)
![(b) Calculate the molar solubility of Ca(OH)2 Ksp = \[Ca2+\]
\[OH-\]2 1.3 x 10-6 = (0.10 + x) (2x)2 (0.10) 4x2 1.3 x 10-5 = 4x2 x =
0.0018M Molar solubility of = 0.0018 M in 0.10 M \[assuming x << O.
10\] I point is earned for the correct stoichiometry and setup. 1
point is earned for the final answer.](media/image235.png)
Question 6
Anion: A Negative ION
CATion: PAWSitive (cats have paws)

Question 7 (a)